Zusammenfassung
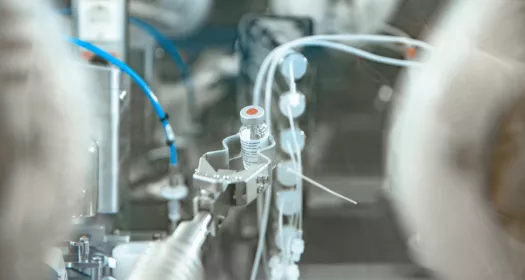
~根据生产计划执行分配的生产任务和活动,使产品能够按照相关的 GMP、安全和环境准则及时生产出保质保量的产品。
~服务运营商
~根据相关的 GMP、安全和环境准则,根据生产计划执行分配的生产任务和活动。
根据生产流程和进度开展日常运营支持活动,实现产品质量和数量的及时生产
~文档专家管理员
~文档专家管理管理 GMP 制造文档和批次记录版本的修改、编辑、分发、审查和归档,以便在质量和期限内将其交付给生产。文档专家管理员确保为该单位的正常运作提供一套必要的行政任务。
About the Role
Major accountabilities:
- Equipment Operator -Participation to the manufacturing processes -Execution of all assigned activities according to production schedule and in compliance with the valid GMP, work, operating, environmental and safety instructions and guidelines -Execution of all assigned activities according to production schedule and in compliance with the valid GMP, work, operating, environmental and safety instructions and guidelines -Execution of all assigned activities according to production schedule and in compliance with the valid GMP, work, operating, environmental and safety instructions and guidelines -Execution of all assigned activities according to production schedule and in compliance with the valid GMP, work, operating, environmental and safety instructions and guidelines -Execution of all assigned activities according to production schedule and in compliance with the valid GMP, work, operating, environmental and safety instructions and guidelines -Execution of all assigned activities according to production schedule and in compliance with the valid GMP, work, operating, environmental and safety instructions and guidelines -Execution of all assigned activities according to production schedule and in compliance with the valid GMP, work, operating, environmental and safety instructions and guidelines -Execution of all assigned activities according to production schedule and in compliance with the valid GMP, work, operating, environmental and safety instructions and guidelines -Execution of all assigned activities in compliance with the GMP, occupational safety and environmental guidelines -Service Operator -Participation to the manufacturing support processes -Execution of all assigned activities according to production schedule and in compliance with the valid GMP, work, operating, environmental and safety instructions and guidelines -Execution of all assigned activities according to production schedule and in compliance with the valid GMP, work, operating, environmental and safety instructions and guidelines -Execution of all assigned activities according to production schedule and in compliance with the valid GMP, work, operating, environmental and safety instructions and guidelines -Execution of all assigned activities according to production schedule and in compliance with the valid GMP, work, operating, environmental and safety instructions and guidelines -Execution of all assigned activities according to production schedule and in compliance with the valid GMP, work, operating, environmental and safety instructions and guidelines -Execution of all assigned activities according to production schedule and in compliance with the valid GMP, work, operating, environmental and safety instructions and guidelines -Execution of all assigned activities according to production schedule and in compliance with the valid GMP, work, operating, environmental and safety instructions and guidelines -Execution of all assigned activities according to productio
Key performance indicators:
- Deadlines: compliance with production planning, execution of tasks on time -Quality: Amount of errors in production documents edited or updated and in batch record review -Time management for shop floor where required
Minimum Requirements:
Work Experience:
- Operations Management and Execution.
- Collaborating across boundaries.
- Functional Breadth.
Skills:
- Transportation.
- General HSE Knowledge.
- Knowledge of GMP.
- Art Curator.
- Transportation Classification (i.e. IATA or DOT) expertise.
- Manufacturing Process Execution.
Languages :
- English.
Why Novartis: Helping people with disease and their families takes more than innovative science. It takes a community of smart, passionate people like you. Collaborating, supporting and inspiring each other. Combining to achieve breakthroughs that change patients’ lives. Ready to create a brighter future together? https://www.novartis.com/about/strategy/people-and-culture
Join our Novartis Network: Not the right Novartis role for you? Sign up to our talent community to stay connected and learn about suitable career opportunities as soon as they come up: https://talentnetwork.novartis.com/network
Benefits and Rewards: Read our handbook to learn about all the ways we’ll help you thrive personally and professionally: https://www.novartis.com/careers/benefits-rewards
无障碍及便利 设 施
诺华 承 诺 与残障人士共事并 为 他 们 提供合理的便利 设 施。如果您由于健康状况或残障 在招聘 过 程的任何 环 节 需要合理便利 设 施 或者 为 了履行 职 位的基本 职 能 请发 送 电 子 邮 件至 [email protected] 告知您的需求和 联 系方式,并在 邮 件中附上您的 职 位申 请编 号。